Essential Guide to Finding Protons, Neutrons, and Electrons in 2025
Understanding Atomic Structure
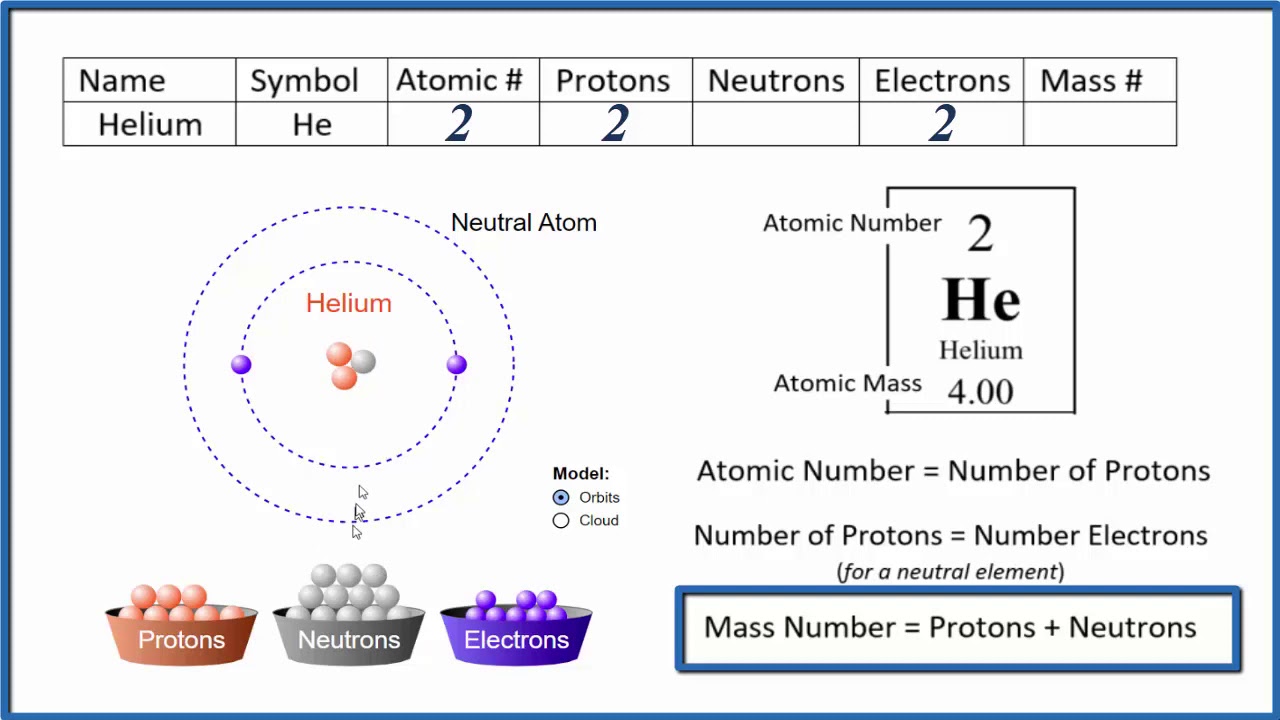
The foundation of chemistry lies in understanding **atomic structure**. Atoms are the building blocks of matter, composed of three fundamental subatomic particles: protons, neutrons, and electrons. To effectively engage with the **atomic theory**, it’s essential to grasp how these particles interrelate and their roles in determining the properties of elements. Every atom has a nucleus made up of protons and neutrons, while electrons orbit the nucleus in an electron cloud. This dynamic structure makes up the essence of *matter composition* and plays a pivotal role in how elements interact chemically.
The Role of Protons in Atoms
Protons are positively charged particles found within the nucleus of an atom. Each element in the **periodic table** is defined by its unique proton count, known as its **atomic number**. The atomic number determines the identity of an element and influences its chemical properties and behavior. For example, hydrogen has one proton, making it the simplest element, while elements like uranium can have over 90 protons. Understanding how to find protons is fundamental for anyone studying **chemistry basics** and *element classification* since shifting the number of protons transforms one element into another.
Neutrons: The Silent Stabilizers
Neutrons, unlike protons, carry no electric charge. Instead, their presence is crucial for the stability of the nucleus. The total number of protons and neutrons gives us the **mass number** of an atom. Moreover, isotopes are variants that share the same atomic number but differ in neutron count. For example, carbon-12 and carbon-14 are isotopes of carbon, where the difference in neutron count influences nuclear stability and *atomic interactions*. To determine neutrons, subtract the atomic number from the mass number—a fundamental method in **calculating atomic particles**.
Electrons: The Charged Players
Electrons, the negatively charged counterparts to protons, orbit the nucleus in specific energy levels or *electron shells*. The relationship between protons and electrons establishes the net charge of an atom—typically, this balance keeps atoms neutral. However, when atoms lose or gain electrons, they form **ions**, which have implications in *chemical reactions* and *atomic properties*. In concepts like electron configuration, understanding how to find electrons is vital in predicting an element’s reactivity and bonding behavior.
Methods of Determining Protons, Neutrons, and Electrons
Several methods and calculations are available for identifying **atomic particles**. To begin with, knowing the **atomic number** provides immediate insight into the proton count. Following this, the **mass number** allows for calculating the neutron count. Lastly, electrons are often inferred based on an atom’s charge and atomic number.
Calculation of Atomic Particles
To effectively determine the number of protons, neutrons, and electrons, we can use straightforward formulas:
1. **Proton Count:** The **atomic number** directly correlates to the proton count.
2. **Neutron Count:** Neutron count can be calculated using the equation: Neutrons = Mass Number – Atomic Number.
3. **Electron Count:** For neutral atoms, the electron count equals the proton count. For ions, subtract or add electrons based on the ion’s charge. This self-contained approach allows for effective **atomic calculations** in various educational and research contexts.
Practical Examples Using the Periodic Table
Using the periodic table enhances our understanding of **element identification**. For example, let’s use oxygen. Oxygen has an atomic number of 8 and a mass number of 16. Based on these figures, we can determine:
– Protons = Atomic Number = 8
– Neutrons = Mass Number – Atomic Number = 16 – 8 = 8
– Electrons (in neutral state) = 8
This method proves essential for understanding **atomic interactions** and supports concepts derived from *element properties* and subatomic behavior.
Nuclear Charge and Stability
Nuclear charge is crucial in comprehending how protons, neutrons, and electrons interact. The positive charge of protons binds the negatively charged electrons through electrostatic forces. Understanding this balance is vital when studying unstable isotopes where variations in neutrons can lead to decay. This foundational knowledge aids in reaching a comprehensive understanding of **nuclear physics fundamentals**, providing a backdrop for *advanced atomic theories* and the implications for particle interaction.
The Importance of Subatomic Particles in Chemistry
Subatomic particles hold significant sway over the properties and reactions of atoms. Thus, grasping their characteristics is critical for aspiring chemists. Chemistry theories revolve around how these particles interact, influencing everything from elemental stability to chemical bonding.
The Electron Configuration in Chemistry
Electron configuration refers to the arrangement of electrons in an atom’s electron shells. This arrangement significantly affects an element’s chemical behavior. *Finding electron configuration* involves understanding the distribution of electrons in accordance with the principles of atomic shells and subshells, namely the Aufbau principle, Pauli exclusion principle, and Hund’s rule. For example, the electron configuration of carbon (C) is 1s² 2s² 2p², indicating how electrons occupy various energy levels, influencing its reactivity and ability to form bonds.
Stability and Isotopes
Isotopes arise from variations in the number of neutrons, showcasing the significance of nuclear stability in atomic behavior. Isotopes play a vital role in both environment impacts and applications such as radiometric dating in archaeological finds. Understanding the function of neutrons in maintaining nuclear stability can redirect research focuses in **particle physics** and **nuclear chemistry**. The nuances of this subject become essential to broader discussions in **chemical nomenclature**.
Connecting Theory to Practical Learning
Applying atomic theory through experiments illustrates the relationship between theory and practice. Engaging in **laboratory exercises** involving element classification and subatomic particle identification can solidify theoretical knowledge and illuminate the intricacies of **atomic structure**. Implementing **effective strategies for chemistry learning** in real-world experiments promotes a deeper understanding and connects theoretical knowledge to observable *atomic behavior*.
Key Takeaways
- Understanding atomic structure involves recognizing the roles of protons, neutrons, and electrons.
- Calculation methods like using atomic and mass numbers are essential for determining subatomic particles.
- The balance of nuclear charge influences stability, affecting atom interactions and properties.
- Isotopes highlight the significance of neutrons, leading to real-world applications in science and technology.
- Engaging in practical chemistry enhances comprehension of atomic theory through experiential learning.
FAQ
1. How do you measure the number of protons in an element?
The number of protons in an element is indicated by its atomic number on the periodic table. Thus, to determine protons, refer to the atomic number directly associated with the element in question.
2. What methods can accurately calculate neutron count?
To accurately calculate neutron count, use the formula: Neutron Count = Mass Number – Atomic Number. This straightforward calculation helps illustrate the relationship between protons, neutrons, and atomic mass.
3. How can electrons impact chemical reactions?
Electrons play a pivotal role in chemical reactions by determining an atom’s reactivity based on **electron configuration**. Atoms seek to reach a stable state, often altering their electron count through gaining or losing electrons during reactions, subsequently forming ions.
4. What are isotopes and why are they important?
Isotopes are variants of elements that differ in neutron count but have the same atomic number. Their significance lies in applications such as radiometric dating and understanding nuclear stability, which reveals crucial insights into the behavior of elements.
5. What is the significance of knowing atomic number in chemistry?
The atomic number signifies the number of protons in an atom, thereby defining the element. Understanding atomic number is paramount for identifying elements, predicting their properties, and establishing seamless connections within **particle physics**.